Benzodiazepines (BZDs) are a class of medications widely prescribed for their effectiveness in treating various conditions, including anxiety, insomnia, and muscle spasms. Since their introduction as safer alternatives to barbiturates, they have become a cornerstone in managing central nervous system excitability. However, with a diverse range of benzodiazepines available, each with unique properties, understanding their differences, particularly in duration of action, is crucial for effective and safe clinical use. This article delves into the world of benzodiazepines, with a specific focus on the longest acting options, exploring their mechanisms, clinical applications, potential side effects, and important considerations for prescribers and patients alike. We aim to provide a comprehensive overview, drawing on established pharmacological principles and clinical insights, to clarify the role of long-acting benzodiazepines in modern medicine.
Benzodiazepine Pharmacology: How They Work
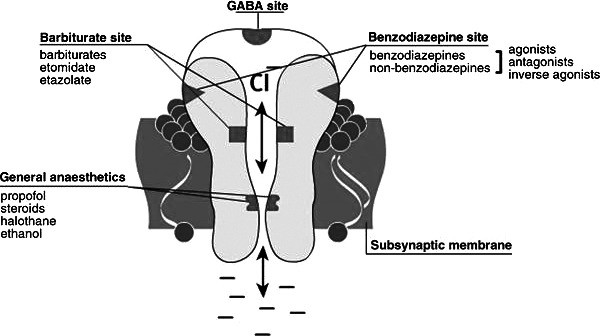
Benzodiazepines exert their effects by modulating the activity of gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the central nervous system. The GABA-A receptor, a chloride ion channel, is the main target for BZDs. GABA’s role is to reduce neuronal excitability, promoting a calming effect in the brain. Benzodiazepines enhance GABA’s effects by acting as positive allosteric modulators at the GABA-A receptor.
The GABA-A receptor is a complex structure composed of five glycoprotein subunits (Figure 1). It features two α subunits, two β subunits, and one γ subunit. Each receptor has two GABA binding sites and a single benzodiazepine binding site located at the interface of the α and γ subunits. Specifically, isoforms 1, 2, 3, and 5 of the α subunit contain a histidine residue that exhibits high affinity for benzodiazepines. When a benzodiazepine molecule binds to this site, it induces a conformational change in the GABA-A receptor. This change facilitates the binding of GABA, which in turn opens the chloride channel, leading to hyperpolarization of the neuron. This hyperpolarization reduces neuronal excitability, resulting in the therapeutic and side effects associated with benzodiazepines.
Figure 1.
Alt text: Diagram of the GABA-A receptor complex showing binding sites for GABA and benzodiazepines, illustrating the mechanism of action for neuronal inhibition.
Specific Benzodiazepine Receptors and Their Effects
The benzodiazepine receptor subtypes, classified based on their α subunit isoforms, mediate different clinical effects. BZ1 receptors, containing the α1 isoform, are predominantly found in the cortex, thalamus, and cerebellum. Activation of BZ1 receptors is primarily responsible for the sedative and hypnotic effects of benzodiazepines, as well as anterograde amnesia and some anticonvulsant actions. Approximately 60% of GABA-A receptors contain the α1 subunit, making amnesia a common side effect. Lipid solubility plays a significant role in amnesia risk, with highly lipid-soluble BZDs exhibiting faster absorption and onset of action, but also a greater risk of amnesia.
BZ2 receptors, containing the α2 isoform, are concentrated in the limbic system, motor neurons, and the dorsal horn of the spinal cord. These receptors mediate the anxiolytic and muscle-relaxant properties of benzodiazepines. The anxiolytic effects are thought to be mediated through BZ2 receptors in the limbic system, while muscle relaxation is achieved via α2-containing receptors in the spinal cord and motor neurons. It’s important to note that benzodiazepines do not interact uniformly with all BZ receptor types or with equal affinity. These variations in receptor affinity and distribution within the central nervous system account for the diverse effects observed across different benzodiazepines.
Pharmacokinetics: Duration of Action Explained
Pharmacokinetics, the study of drug movement within the body, dictates the onset and duration of a benzodiazepine’s effects. It encompasses absorption, distribution, metabolism, and excretion. Benzodiazepines can be administered through various routes, including oral, intravenous, intramuscular, sublingual, intranasal, and rectal. Drug characteristics like lipid solubility, plasma protein binding, and molecular size influence their distribution volume. Pharmacodynamics, in contrast, describes how drugs affect the body, focusing on receptor responsiveness and mechanisms of action. Individual responses to benzodiazepines vary due to differences in pharmacokinetics and pharmacodynamics.
Elimination half-life, a crucial pharmacokinetic parameter, is the time it takes for the plasma concentration of a drug to reduce by 50% during elimination. It is directly proportional to the volume of distribution and inversely proportional to clearance. Liver and kidney diseases, which alter distribution or clearance, significantly affect elimination half-life. It’s important to understand that elimination half-life doesn’t directly represent the time to recovery from drug effects, but rather the time for plasma concentration to halve. Generally, a drug is considered almost entirely eliminated after approximately five half-lives. Drug accumulation can occur if dosing intervals are shorter than this period.
Benzodiazepines are generally well-absorbed from the gastrointestinal tract after oral administration. Intravenous administration leads to rapid distribution to the brain and central nervous system. Termination of benzodiazepine action, similar to lipid-soluble barbiturates, often occurs through redistribution. Intramuscular absorption of diazepam and chlordiazepoxide can be slow and erratic, while lorazepam and midazolam show more rapid and complete absorption via intramuscular routes. Lorazepam is also effectively absorbed sublingually, reaching peak levels in about 60 minutes.
Benzodiazepines and their metabolites are highly protein-bound and widely distributed, accumulating in lipid-rich tissues like the central nervous system and adipose tissue. Lipophilic benzodiazepines generally exhibit faster absorption and onset of effects. Metabolism primarily occurs in the liver through cytochrome P450 enzymes (phase I) followed by glucuronide conjugation (phase II), with excretion mainly in the urine. Notably, some benzodiazepines produce active metabolites, which can prolong their overall duration of action. Diazepam, a long-acting benzodiazepine, is a prime example, producing active metabolites like oxazepam, desmethyldiazepam, and temazepam, significantly extending its effects, especially concerning for elderly patients or those with hepatic impairment. Midazolam, a short-acting benzodiazepine, does not produce active metabolites.
Benzodiazepines in Clinical Practice: Focusing on Duration
Benzodiazepines are clinically categorized based on their elimination half-life: short-acting (1-12 hours), intermediate-acting (12-40 hours), and long-acting (40-250 hours). Considering that approximately five half-lives are needed for complete elimination, the actual duration of drug presence in the body is considerably longer than the half-life itself. The following table summarizes commonly prescribed benzodiazepines and their characteristics.
Table.
Benzodiazepines Commonly Prescribed in Clinical Practice
Alt text: Table listing commonly prescribed benzodiazepines, categorized by brand name, half-life, and typical uses in clinical practice, highlighting variations in duration of action.
Benzodiazepines are also characterized by their relative potency. Early benzodiazepines like chlordiazepoxide, oxazepam, and temazepam were of low to medium potency and long-acting. Their effectiveness and relatively low toxicity made them first-line treatments for anxiety and insomnia. Subsequently, high-potency benzodiazepines such as alprazolam, lorazepam, and clonazepam were developed, expanding their applications to panic disorders, obsessive-compulsive disorder (as adjuncts to SSRIs), and acute mania or agitation (as adjuncts to antipsychotics). While newer, high-potency benzodiazepines offer improved therapeutic effects and faster onset, they also carry a higher risk of adverse effects. Therefore, clinicians must carefully consider individual pharmacokinetic properties when prescribing any benzodiazepine.
Longest Acting Benzodiazepines: Diazepam, Chlordiazepoxide, and Flurazepam
When considering the “Longest Acting Benzo”, several benzodiazepines fall into this category, primarily due to their long elimination half-lives and the presence of active metabolites. Diazepam, chlordiazepoxide, and flurazepam are among the most prominent examples of long-acting benzodiazepines.
Diazepam: As a long-acting, medium-potency benzodiazepine (Figure 6), diazepam is versatile, used for anxiety, sedation, muscle relaxation, and as an anticonvulsant. It interacts with equal affinity across all BZD-sensitive receptors in the central nervous system. Its anxiolytic effects manifest at lower doses via α2-containing receptors in the limbic system, while muscle relaxation occurs at higher doses through α2-receptors in the spinal cord and motor neurons, and to a lesser extent, α3-receptors. Sedation and anterograde amnesia at higher doses are mediated by α1-receptors. A key feature of diazepam is its hepatic metabolism into active metabolites: oxazepam, temazepam, and desmethyldiazepam. These metabolites contribute significantly to its prolonged elimination half-life, which can increase by approximately one hour for each year of age over 40. This extended duration and potential for metabolite accumulation necessitate caution, particularly in elderly patients and those with hepatic or renal dysfunction, due to risks of oversedation and prolonged amnesia. Intravenous diazepam formulations require propylene glycol for water solubility, which can cause injection pain and thrombophlebitis. For anxiety, oral diazepam dosages range from 2-10 mg, 2-4 times daily. Intramuscular and intravenous forms are also available for acute anxiety. For seizures and muscle relaxation, oral doses of 2-10 mg up to four times daily are common. In status epilepticus, initial intravenous doses can be 5-10 mg, repeated if necessary.
Figure 6.
Alt text: Chemical structure of diazepam, a long-acting benzodiazepine, highlighting its molecular composition relevant to its pharmacological properties.
Chlordiazepoxide: As the first benzodiazepine discovered, chlordiazepoxide is also long-acting and of low to medium potency. It is used for anxiety, alcohol withdrawal, and as a sedative. Similar to diazepam, its long duration is attributed to active metabolites. While effective, it is less commonly used today compared to newer benzodiazepines, but remains relevant, particularly in managing alcohol withdrawal symptoms due to its gradual onset and sustained effect, which can help mitigate withdrawal symptoms over a longer period.
Flurazepam: Flurazepam is another long-acting benzodiazepine primarily indicated for insomnia. Its long half-life and active metabolites ensure a prolonged duration of action, aiming to maintain sleep throughout the night. However, this long duration can also lead to daytime sedation and accumulation, especially in older adults. Due to the risk of next-day impairment and the availability of shorter-acting hypnotics, flurazepam is less favored for routine insomnia management compared to more recently developed options with shorter durations of action.
Shorter-Acting Benzodiazepines: Alprazolam, Clonazepam, Lorazepam, and Midazolam
In contrast to long-acting options, shorter-acting benzodiazepines like alprazolam, clonazepam, lorazepam, and midazolam offer different clinical profiles.
Alprazolam: Alprazolam (Figure 2) is a short-acting, high-potency benzodiazepine, with a shorter elimination half-life compared to diazepam. It is widely used for panic disorders and anxiety. Typical doses for anxiety start at 0.25-0.5 mg three times daily, with a maximum daily dose of 4 mg. For panic disorders, doses may reach 6-10 mg daily. A significant concern with alprazolam is rebound anxiety upon abrupt discontinuation due to its short half-life.
Figure 2.
Alt text: Chemical structure of alprazolam, a short-acting, high-potency benzodiazepine commonly used for anxiety and panic disorders.
Clonazepam: Clonazepam (Figure 3) is a high-potency, long-acting benzodiazepine, although often categorized as intermediate-acting in some clinical contexts due to its half-life being shorter than diazepam. It possesses both GABA-A receptor agonist and serotonin agonist properties. Clinically, it’s used as an anticonvulsant and anxiolytic. Studies have shown it to be effective for acute mania and panic disorder. Its longer half-life compared to alprazolam reduces the risk of rebound anxiety upon discontinuation. Lower lipid solubility compared to other high-potency BZDs may result in a lower risk of anterograde amnesia. Initial doses for panic disorder are 0.25 mg twice daily, increasing to 0.5 mg twice daily, with a maximum of 1-4 mg daily. For seizure disorders, adult starting doses are 0.5 mg three times daily, up to a maximum of 20 mg daily. Pediatric dosing starts at 0.01-0.03 mg/kg daily in 2-3 divided doses, up to 0.1-0.2 mg/kg daily.
Figure 3.
Alt text: Chemical structure of clonazepam, an intermediate to long-acting, high-potency benzodiazepine used for anxiety and seizure disorders.
Lorazepam: Lorazepam (Figure 4) is another high-potency, short-acting benzodiazepine. It is slightly less lipid-soluble than alprazolam, potentially reducing amnesia risk. It binds GABA-A receptors with less affinity than alprazolam but more than clonazepam. Effective as an anticonvulsant and adjunct in acute agitation and mania, lorazepam is rapidly and completely absorbed intramuscularly and sublingually. Unique in its direct glucuronidation without cytochrome P450 metabolism, lorazepam is relatively safe in patients with hepatic or renal dysfunction. For alcohol withdrawal, typical dosing is 2 mg every 6 hours for four doses, then 1 mg every 6 hours for eight doses. For anxiety, 2-3 mg daily in divided doses, up to a maximum of 10 mg daily, is common. For ICU sedation, intravenous infusion rates of 0.01-0.1 mg/kg/h are used.
Figure 4.
Alt text: Chemical structure of lorazepam, a short-acting, high-potency benzodiazepine known for its versatility and use in patients with liver impairment.
Midazolam: Midazolam (Figure 5) is a short-acting benzodiazepine, approximately 1.5-2 times more potent than diazepam with greater hypnotic effects due to GABA reuptake interference. Primarily used preoperatively for sedation and anxiolysis, it is available in various formulations (IV, IM, oral, sublingual, rectal, intranasal). Its water solubility at acidic pH (parenteral form) reduces injection pain compared to diazepam. However, at physiological pH, it becomes highly lipophilic, leading to rapid absorption, blood-brain barrier penetration, and quick onset of action. Rapid redistribution results in a short duration and elimination half-life, making it suitable for continuous infusion. Midazolam can cause peripheral vasodilation and blood pressure decrease, more pronounced than with diazepam. Metabolized into inactive 1-hydroxymidazolam, anterograde amnesia is often a desired effect, especially in surgical settings. Preoperative sedation doses range from 1-5 mg IV up to one hour before surgery in healthy patients, and lower doses (up to 3 mg) for higher-risk patients. Pediatric oral syrup doses up to 1 mg/kg (max 20 mg) for children over 6 months, and IM/intranasal doses of 0.1-0.5 mg/kg (max 10 mg) are used.
Figure 5.
Alt text: Chemical structure of midazolam, a very short-acting benzodiazepine primarily used for procedural sedation and anesthesia induction.
Side Effects and Safety Considerations
Common side effects across all benzodiazepines include drowsiness, lethargy, and fatigue. Higher doses can lead to impaired motor coordination, dizziness, vertigo, slurred speech, blurred vision, mood swings, euphoria, and in some cases, hostile or erratic behavior. Due to slow elimination, repeated doses can cause significant accumulation in fatty tissues, leading to symptoms of overmedication like impaired thinking, disorientation, confusion, and slurred speech over time. Tolerance, dependence, and withdrawal are also significant concerns with long-term use.
Drug interactions are a crucial consideration. Benzodiazepines are metabolized by the cytochrome P450 system in the liver. Drugs that inhibit (e.g., oral contraceptives, antifungals, some antibiotics) or induce (e.g., carbamazepine, phenytoin, rifampin, St. John’s wort) these enzymes can alter benzodiazepine elimination half-life, increasing or decreasing drug effects, respectively.
Severe adverse effects can occur with concomitant use of benzodiazepines and other central nervous system depressants like opioids. This combination can significantly enhance cardiovascular and hemodynamic perturbations, and dramatically increase respiratory depression, especially in patients with chronic obstructive pulmonary disease. Respiratory depressant effects are dose-dependent and synergistic.
Venous irritation can occur with intravenous diazepam and lorazepam.
Age-Related Pathophysiologic Changes and Benzodiazepine Use
Aging is associated with a decline in homeostatic mechanisms, particularly in the central nervous system, liver, and kidneys. Neurological changes include neuron loss, glial cell proliferation, decreased intracellular enzymes, and reduced dendritic synapses. Liver function decline prolongs drug clearance, and renal function decreases by approximately 1% per year after age 40. These age-related changes significantly impact benzodiazepine pharmacokinetics, leading to increased accumulation and sensitivity in the elderly. Elderly individuals often exhibit greater confusion, disorientation, and intensity and duration of benzodiazepine effects due to these factors.
Benzodiazepine-Induced Central Nervous System Toxicity and Altered States
Cognitive Impairment and Amnesia
Cognitive impairment from benzodiazepines encompasses anterograde amnesia, sedation, drowsiness, motor impairment, inattentiveness, and ataxia, often more pronounced in the elderly. The “Beers List” highlights benzodiazepines as potentially hazardous to the elderly due to cognition-impairing effects from drug accumulation. Cognitive impairment increases risks of falls, fractures, and motor vehicle accidents, major causes of injury and death in older adults.
Benzodiazepines primarily affect long-term memory, specifically episodic memory (memory of personal events), while semantic memory (knowledge of facts) is less impacted. Anterograde amnesia, memory loss for events after drug administration, is a well-documented effect. While used intentionally for amnesia in perioperative settings, it is an undesired side effect in most other cases. Age-related organ decline exacerbates amnesia risk due to reduced drug metabolism and elimination, leading to potential toxicity. Even standard benzodiazepine doses can cause significant memory loss and confusion as drugs accumulate in fatty tissues.
Benzodiazepine-induced amnesia is also a factor in drug-facilitated sexual assault (DFSA). Benzodiazepines, sometimes in combination with alcohol, can impair victim’s memory of assault events, complicating prosecution and victim recovery.
Disinhibition and Delirium
Disinhibition, another consequence of benzodiazepine accumulation, can lead to impulsive and risky behaviors, such as high-risk sexual activity and reckless driving. Benzodiazepine use has been linked to an increased risk of motor vehicle accidents. Studies show impaired driving performance even after single doses, and these effects can persist with daily use, despite potential tolerance development.
Delirium, characterized by impaired attention and cognition, is another serious concern, especially in intensive care settings. Benzodiazepines increase delirium risk, particularly in elderly ICU patients, with incidence rates ranging from 78% to 87% in some studies. Delirium is associated with increased morbidity, mortality, and longer hospital stays, partly due to increased risk of nosocomial infections. Benzodiazepine use prior to ICU admission has also been linked to early delirium onset.
Conclusion: Prudent Use of Benzodiazepines
Benzodiazepines are valuable medications for various conditions, but their use requires careful consideration of dose-related side effects, drug interactions, and patient-specific factors, especially age and comorbidities. Long-acting benzodiazepines like diazepam, chlordiazepoxide, and flurazepam offer prolonged therapeutic effects but carry a higher risk of accumulation and prolonged side effects, particularly in vulnerable populations. Shorter-acting benzodiazepines may be preferable in many situations to minimize accumulation and next-day effects. Clinicians must carefully weigh the benefits and risks before prescribing any benzodiazepine, considering safer alternatives when appropriate and educating patients about potential side effects and risks associated with these medications.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.