Soma®, known generically as carisoprodol, is a muscle relaxant that has gained attention for its therapeutic use and, alarmingly, its abuse potential. A common question arises when discussing Soma: Is Soma A Benzo? This article aims to clarify the nature of carisoprodol, differentiate it from benzodiazepines, and delve into its mechanism of action, abuse risks, and regulatory status. Understanding these aspects is crucial for healthcare professionals and the public alike, especially given the rising concerns surrounding carisoprodol abuse.
What is Soma (Carisoprodol)?
Carisoprodol was approved in 1959 as a muscle relaxant and marketed under the brand name Soma®. It’s prescribed for short-term relief of musculoskeletal pain and discomfort, often used in conjunction with rest and physical therapy. Carisoprodol is also available in combination with analgesics like aspirin or codeine to enhance pain relief. Despite its clinical utility, carisoprodol’s mechanism of action and potential for abuse have become significant areas of research and concern.
Soma vs. Benzodiazepines: Key Differences
To directly address the question, Soma is not a benzodiazepine. Benzodiazepines, such as Valium® (diazepam) and Xanax® (alprazolam), are a class of psychoactive drugs with sedative, anxiety-reducing, and muscle-relaxant properties. They work primarily by enhancing the effects of the neurotransmitter gamma-aminobutyric acid (GABA) in the brain, specifically at benzodiazepine binding sites on GABA receptors (GABAARs).
Carisoprodol, on the other hand, belongs to a class of drugs known as carbamates. While it also exerts muscle relaxant and sedative effects, its mechanism is distinct from benzodiazepines. The confusion may arise because both can depress the central nervous system (CNS) and have sedative properties, leading to overlapping recreational use and abuse patterns. However, at a molecular level, they are different drugs acting through somewhat different pathways.
Mechanism of Action: How Soma Works
The precise mechanism of action of carisoprodol is not fully understood, but research has shed light on its GABAergic properties. Initially, it was believed that carisoprodol’s effects were primarily due to its metabolism into meprobamate. Meprobamate is an anxiolytic and sedative drug, classified as a Schedule IV controlled substance, known to act on GABAARs, similar to barbiturates.
However, recent studies indicate that carisoprodol itself possesses significant CNS activity, independent of meprobamate. Carisoprodol has been shown to modulate GABAAR function directly, in a manner similar to CNS depressants like barbiturates. This intrinsic GABAergic activity of carisoprodol may explain its therapeutic effects and, crucially, contribute to its abuse potential.
Research suggests that carisoprodol, like barbiturates, can allosterically modulate and directly activate GABAARs. This means it can enhance the receptor’s response to GABA and, at higher concentrations, directly activate the receptor even in the absence of GABA. Interestingly, studies have shown that carisoprodol can be more potent and efficacious than meprobamate in these GABAergic actions. Unlike benzodiazepines which require the γ2 subunit of the GABAAR for their potentiation, carisoprodol, similar to barbiturates, can regulate GABAAR function even without this subunit. However, carisoprodol does not interact with the same specific sites on the GABAAR as benzodiazepines or barbiturates, indicating a unique, though related, mechanism.
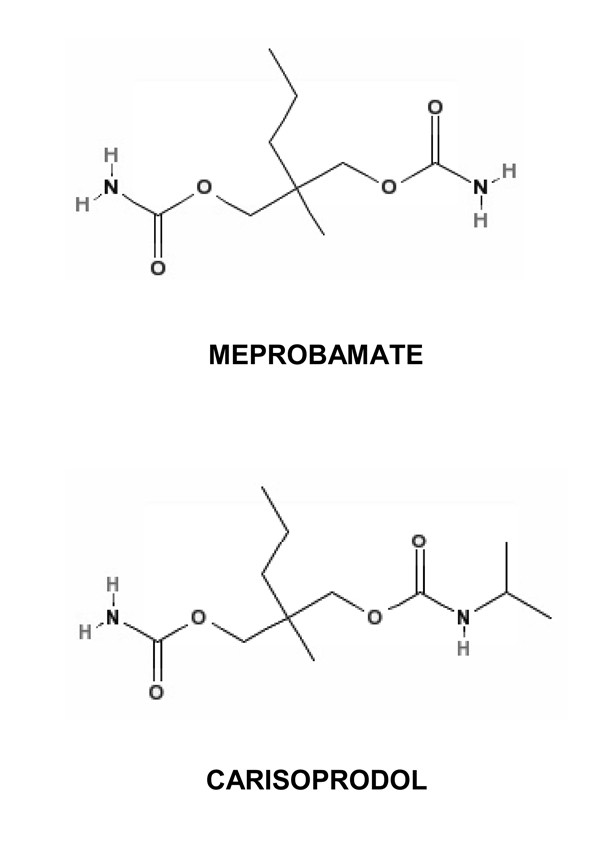 Chemical structures of carisoprodol and meprobamate
Chemical structures of carisoprodol and meprobamate
Figure 1: Chemical structures of carisoprodol and meprobamate, highlighting their structural similarity as propanediol dicarbamates.
Abuse Potential and Risks of Soma
Despite initial assumptions that carisoprodol had low abuse potential, widespread reports and studies have documented significant abuse, dependence, and withdrawal issues. Carisoprodol abuse is a growing concern, with the Drug Abuse Warning Network ranking it among the most abused drugs, even surpassing substances like oxycodone and methadone in some reports.
Carisoprodol is often misused to intensify the effects of other CNS depressants, including opioids and alcohol, or to manage opioid withdrawal symptoms. Its easy availability, particularly through online pharmacies that may operate without requiring prescriptions, and relatively low cost have likely contributed to its increasing abuse. The consequences of carisoprodol abuse can be severe, including tolerance, dependence, withdrawal syndromes, overdose, and even fatalities. Reports from various regions, including the US and Europe, highlight the escalating problem of carisoprodol abuse and related deaths.
Why the Confusion? Soma’s Sedative Effects
The sedative properties of carisoprodol, while not mediated through the same specific receptor sites as benzodiazepines, contribute to the confusion. Both benzodiazepines and carisoprodol can cause drowsiness, relaxation, and reduced anxiety, especially at higher doses. This overlap in subjective effects might lead individuals to mistakenly categorize Soma as a benzodiazepine, particularly when seeking information online or through informal sources. Furthermore, in polydrug abuse scenarios, individuals may not always differentiate between the specific drugs they are using, focusing instead on the overall desired effects of sedation or euphoria.
Regulatory Status and the Need for Re-evaluation
A critical point of concern is the regulatory status of carisoprodol. While meprobamate, its primary metabolite, is a Schedule IV controlled substance, carisoprodol itself is not federally scheduled in the United States. This discrepancy is paradoxical given the evidence that carisoprodol itself has intrinsic CNS depressant activity and a significant abuse liability, potentially exceeding that of meprobamate.
Several states in the US have recognized the danger and classified carisoprodol as a Schedule IV controlled substance. Internationally, the European Medicines Agency recommended suspending marketing authorization for carisoprodol-containing products due to its abuse potential outweighing therapeutic benefits.
The FDA’s eight-factor analysis for drug scheduling considers factors like abuse potential, history of abuse, public health risk, and pharmacological evidence. Growing evidence, including recent research on carisoprodol’s direct GABAergic effects, strongly suggests that carisoprodol meets several of these criteria for scheduling. Re-evaluating the non-scheduled status of carisoprodol at the federal level is increasingly warranted to mitigate its escalating abuse and associated harms.
Treatment for Soma Dependence and Overdose
Treatment for carisoprodol dependence and withdrawal is challenging. Currently, it often involves managing withdrawal symptoms with benzodiazepines or barbiturates to address anxiety and insomnia. However, carisoprodol overdose presents unique complications, often characterized by agitation and seizures, which can be exacerbated by typical CNS depressant overdose treatments. Supportive therapy remains a primary approach in overdose cases.
A deeper understanding of carisoprodol’s mechanism of action is crucial for developing more targeted and effective treatments for dependence, withdrawal, and overdose. Identifying specific sites of action and subunit-dependent effects on GABAARs may pave the way for novel therapeutic agents with reduced abuse potential and improved treatment strategies for carisoprodol misuse.
Conclusion
In conclusion, while Soma (carisoprodol) shares some sedative properties with benzodiazepines, it is not a benzo. Carisoprodol is a distinct muscle relaxant with its own mechanism of action, primarily involving direct modulation of GABA receptors in a manner similar to barbiturates. Its significant abuse potential, coupled with its current non-scheduled status at the federal level in the US, raises serious concerns. The rising tide of carisoprodol abuse necessitates increased awareness among healthcare providers and the public, along with a critical re-evaluation of its regulatory classification to protect public health and safety.
References