INTRODUCTION
Benzodiazepines (BZDs), while not directly related to automotive repair at first glance, are a class of medications with significant implications across various fields, including scenarios where automotive professionals might encounter individuals affected by them. Understanding Benzos Classification, their mechanisms, effects, and potential side effects is crucial for a well-rounded understanding of human factors that can indirectly impact even industries like automotive service. Originally developed as safer alternatives to barbiturates, BZDs have become widely prescribed for a range of conditions, from anxiety and insomnia to muscle spasms and seizures. This article provides an in-depth exploration of benzos classification, delving into their pharmacology, clinical applications, and the importance of recognizing their effects.
BENZODIAZEPINE PHARMACOLOGY: Understanding the Basics of Benzos Classification
General Pharmacodynamics: How Benzos Work
At their core, benzodiazepines exert their effects by acting as positive allosteric modulators of the gamma-aminobutyric acid (GABA)-A receptor. The GABA-A receptor is a crucial component of the central nervous system, functioning as a ligand-gated chloride channel. GABA itself is the brain’s primary inhibitory neurotransmitter, meaning it dampens neuronal excitability and promotes a calming effect. This inhibitory action of GABA is enhanced by benzos. There are three types of GABA receptors (A, B, and C), but BZDs specifically target the GABA-A receptor.
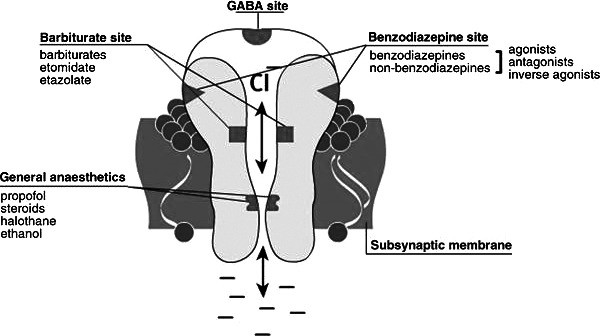
The GABA-A receptor is a complex structure composed of five glycoprotein subunits (as illustrated in Figure 1). Typically, it consists of two α subunits, two β subunits, and one γ subunit. While there are two GABA-binding sites per receptor complex, there’s only one benzodiazepine binding site. This BZD binding site is located at the interface between the α and γ subunits. Notably, specific isoforms within the α subunit (α1, α2, α3, and α5) contain a histidine residue that exhibits a high affinity for BZDs. In contrast, isoforms α4 and α6 contain an arginine residue and do not bind BZDs.
Figure 1. Gamma amino butyric acid receptor with target sites. Adapted from http://ccforum.com/content/12/S3/S4/figure/F1. Alt text: Diagram illustrating the GABA-A receptor complex with labeled target sites for GABA and benzodiazepines, highlighting the subunit composition (alpha, beta, gamma) and the chloride ion channel.
When a benzodiazepine molecule binds to its specific site on the GABA-A receptor, it induces a conformational change. This change makes it easier for GABA to bind to its own site. The enhanced GABA binding then further alters the receptor, opening the chloride channel more effectively. The influx of chloride ions into the neuron hyperpolarizes the cell membrane, making it less likely to fire an action potential. This hyperpolarization is the mechanism behind GABA’s inhibitory effects throughout the central nervous system and how BZDs amplify this calming action.
Specific Benzodiazepine Receptors and Benzos Classification by Receptor Affinity
Further refining benzos classification, the BZD receptor itself is categorized into subtypes based on the α subunit isoforms and their associated clinical effects. This sub-classification helps explain the varied effects of different benzodiazepines.
-
BZ1 Receptors (α1 Subunit): Predominantly found in the cortex, thalamus, and cerebellum, BZ1 receptors are largely responsible for the sedative effects of benzodiazepines. They also contribute to anterograde amnesia and some anticonvulsant effects, such as those seen with diazepam. Approximately 60% of GABA-A receptors contain the α1 subunit, explaining why amnesia is a common side effect. Lipid solubility plays a significant role in amnesia risk; higher lipid solubility correlates with increased amnesia risk due to faster absorption and onset of action.
-
BZ2 Receptors (α2 Subunit): Concentrated in areas like the limbic system, motor neurons, and the dorsal horn of the spinal cord, BZ2 receptors primarily mediate the anxiolytic and muscle-relaxant effects of BZDs. The anxiolytic effects are thought to stem from BZ2 receptors in the limbic system, while muscle relaxation is mediated via α2-containing receptors in the spinal cord and motor neurons.
It is critical to note that not all benzodiazepines interact with the same receptor subtypes or with equal affinity. These variations in α subunit isoforms, receptor affinity, and location within the central nervous system are key factors in the diverse clinical profiles and effects observed across different benzodiazepines, leading to different aspects of benzos classification.
Benzodiazepine Pharmacokinetics: How the Body Handles Benzos
Pharmacokinetics, describing “what the body does to the drug,” is crucial for understanding benzos classification based on duration of action and onset. It encompasses absorption, distribution, metabolism, and excretion (ADME). Pharmacodynamics, conversely, describes “what the drug does to the body,” focusing on receptor responsiveness and mechanisms of action. Individual responses to BZDs vary based on both pharmacokinetic and pharmacodynamic differences.
-
Administration and Absorption: BZDs can be administered through various routes: intramuscular, intravenous, oral, sublingual, intranasal, or rectal. Lipid solubility, plasma protein binding, and molecular size influence drug distribution. More lipophilic BZDs are generally absorbed faster and have a quicker onset of action. Oral administration generally leads to good gastrointestinal absorption. Intravenous administration results in rapid distribution to the brain and CNS. Intramuscular absorption of diazepam and chlordiazepoxide can be slow and erratic, while lorazepam and midazolam are absorbed more rapidly and completely intramuscularly. Lorazepam is also well-absorbed sublingually.
-
Distribution: BZDs and their metabolites are highly protein-bound and distribute widely throughout the body, accumulating in lipid-rich tissues like the CNS and adipose tissue.
-
Metabolism and Excretion: Most BZDs are primarily metabolized oxidatively by cytochrome P450 enzymes (Phase I), followed by glucuronide conjugation (Phase II), and are largely excreted in the urine. However, some BZDs, like lorazepam, undergo direct glucuronidation, bypassing cytochrome P450 metabolism, which can be advantageous in patients with hepatic dysfunction.
-
Active Metabolites: A significant aspect of benzos classification and clinical effect is the presence of active metabolites. Some BZDs, like diazepam, produce active metabolites (oxazepam, desmethyldiazepam, temazepam) that prolong drug action. Midazolam, a short-acting BZD, does not produce active metabolites. The presence of active metabolites is particularly important in elderly patients and those with liver disease, as it can lead to prolonged effects and accumulation.
-
Elimination Half-Life: Elimination half-life, the time for plasma concentration to reduce by 50%, is a key pharmacokinetic parameter used in benzos classification. It’s influenced by volume of distribution and clearance, both of which can be affected by renal and hepatic disease. It takes approximately 5 half-lives for a drug to be nearly eliminated from the body. Therefore, dosing intervals shorter than this can lead to drug accumulation.
BENZODIAZEPINES IN CLINICAL PRACTICE: Benzos Classification by Duration of Action and Potency
General Clinical Use and Benzos Classification by Half-Life
Benzos classification in clinical practice often relies on elimination half-life, which directly impacts the duration of their effects. This classification helps guide appropriate selection for different clinical needs and patient populations.
-
Short-Acting Benzos: Elimination half-life of 1-12 hours. Examples include alprazolam, lorazepam, and midazolam.
-
Intermediate-Acting Benzos: Elimination half-life of 12-40 hours. Examples include temazepam.
-
Long-Acting Benzos: Elimination half-life of 40-250 hours. Examples include diazepam and chlordiazepoxide.
[Table of Benzodiazepines Commonly Prescribed in Clinical Practice]
Table: Benzodiazepines Commonly Prescribed in Clinical Practice. Alt text: Table listing commonly prescribed benzodiazepines with their brand names, available forms (oral, IV, etc.), usual daily dose ranges for adults, and elimination half-lives categorized as short, intermediate, or long-acting.
Another important aspect of benzos classification is relative potency. Early BZDs like chlordiazepoxide, oxazepam, and temazepam were low to medium potency. Later, high-potency BZDs such as alprazolam, lorazepam, and clonazepam were developed. High-potency BZDs often offer faster onset and enhanced therapeutic effects but also carry a greater risk of side effects, requiring careful consideration of individual pharmacokinetic properties when prescribing.
Specific Benzodiazepines: Examples within Benzos Classification
- Alprazolam: A short-acting, high-potency BZD (Figure 2) primarily used for panic disorders and anxiety. It has an elimination half-life of 6-27 hours. A common issue is rebound anxiety upon abrupt discontinuation due to its short half-life.
Figure 2. Chemical structure of alprazolam. Alt text: Structural diagram of the alprazolam molecule, highlighting its chemical formula and arrangement of atoms, relevant to pharmaceutical chemistry and drug identification.
- Clonazepam: Another high-potency, but long-acting BZD (Figure 3) with both GABA-A and serotonin agonist activity. It has anticonvulsant and anxiolytic effects and is effective for panic disorders and acute mania. Its longer half-life reduces rebound anxiety, and lower lipid solubility may reduce anterograde amnesia compared to other high-potency BZDs.
Figure 3. Chemical structure of clonazepam. Alt text: Graphical representation of the molecular structure of clonazepam, showing the bonds and atoms that constitute its chemical makeup for scientific and medicinal context.
- Lorazepam: A short-acting, high-potency BZD (Figure 4) with slightly lower lipid solubility than alprazolam, potentially reducing amnesia risk. It is effective as an anticonvulsant and adjunct to antipsychotics. Notably, lorazepam undergoes direct glucuronidation, making it potentially safer in patients with hepatic dysfunction.
Figure 4. Chemical structure of lorazepam. Alt text: Image depicting the chemical structure of lorazepam, used in pharmaceutical and chemical discussions to visually represent its molecular composition.
- Midazolam: A short-acting BZD (Figure 5) with high potency, primarily used preoperatively for sedation and anxiolysis due to its rapid onset and short duration. Its water solubility at acidic pH and lipophilicity at physiological pH contribute to its rapid action and reduced injection site irritation compared to diazepam.
Figure 5. Chemical structure of midazolam. Alt text: Diagram outlining the chemical structure of midazolam, a benzodiazepine, utilized in chemistry and pharmacology for structural analysis and drug development understanding.
- Diazepam: A long-acting, medium-potency BZD (Figure 6) used for anxiety, sedation, muscle relaxation, and as an anticonvulsant. It interacts with all BZD-sensitive receptors and produces active metabolites, contributing to its long half-life and potential for accumulation, especially in the elderly.
Figure 6. Chemical structure of diazepam. Alt text: Molecular structure of diazepam, displayed as a chemical diagram for reference in scientific and pharmaceutical contexts, indicating atom types and bonds.
Side Effects of Benzodiazepines: Important Considerations Beyond Benzos Classification
Common side effects across all benzos classification categories include drowsiness, lethargy, fatigue, impaired motor coordination, dizziness, and slurred speech. Higher doses can lead to mood swings, euphoria, and even erratic behavior. Accumulation with repeated dosing can cause confusion and impaired thinking. Tolerance, dependence, and withdrawal are significant concerns with long-term use.
Drug interactions are also important, as BZDs are metabolized by the cytochrome P450 system. Drugs affecting these enzymes can alter BZD elimination half-life. Severe adverse effects can occur when BZDs are combined with other CNS depressants like opioids, leading to enhanced respiratory depression and cardiovascular effects.
AGE-RELATED PATHOPHYSIOLOGIC CHANGES AND BENZODIAZEPINES
Aging significantly affects drug metabolism and sensitivity. Reduced function in the CNS, liver, and kidneys in older adults leads to prolonged drug clearance and increased sensitivity to BZDs. This increased sensitivity is due to BZD accumulation and can result in greater confusion, disorientation, and intensified side effects. Therefore, benzos classification by half-life is particularly relevant in elderly patients, with longer-acting agents posing a higher risk of accumulation and adverse effects.
BENZODIAZEPINE-INDUCED CENTRAL NERVOUS SYSTEM TOXICITY AND ALTERED STATES
Cognitive Impairment: A Spectrum of Effects
Cognitive impairment, a broad term encompassing anterograde amnesia, sedation, motor impairment, and ataxia, is a significant concern with BZD use, especially in the elderly. BZDs are listed on the Beers List of medications potentially inappropriate for older adults due to their cognitive effects. Cognitive impairment increases the risk of falls, fractures, and motor vehicle accidents.
Anterograde Amnesia: Memory and Benzos Classification
BZDs primarily impair long-term memory, specifically episodic memory (memory of personal events). Anterograde amnesia (memory loss for events after drug administration) is a well-documented effect. While sometimes desired in perioperative settings, it’s usually an unwanted side effect. The risk is heightened in the elderly due to reduced drug metabolism. The amnesic effects can have serious consequences, including increased vulnerability to sexual abuse, particularly in cases of drug-facilitated sexual assault (DFSA).
Disinhibition and Delirium: Further Risks
Disinhibition, another concerning effect of BZD toxicity, can lead to impulsive and risky behaviors, such as reckless driving and high-risk sexual activity. Studies have linked BZD use to an increased risk of motor vehicle accidents. Delirium, an acute state of confusion and impaired attention, is another serious adverse effect, particularly in hospitalized and elderly patients, increasing morbidity and mortality. BZDs can increase the risk of delirium, especially in the ICU setting.
CONCLUSION: Responsible Use and Understanding Benzos Classification
Benzodiazepines remain valuable medications for various conditions, but their use requires careful consideration of risks and benefits. Understanding benzos classification by half-life, potency, and receptor affinity, along with pharmacokinetic and pharmacodynamic properties, is essential for clinicians. Patient-specific factors, especially age and co-morbidities, must be carefully evaluated. The potential for side effects, drug interactions, and CNS toxicity necessitates prudent prescribing and monitoring to ensure clinically appropriate and safe BZD use.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.